Abstract
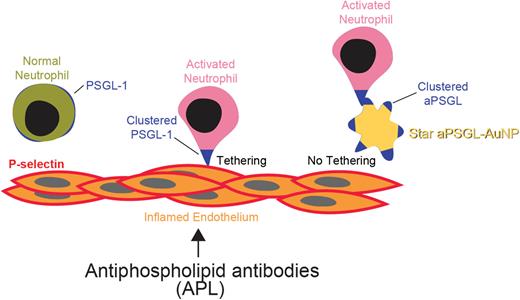
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by recurrent arterial, venous and microvascular thrombosis in the presence of persistently positive antiphospholipid antibodies (aPLs). It often affects young individuals and is a chronic risk factor of myocardial infarction, ischemic stroke, recurrent fetal loss, and multi-organ failure. Presently thrombosis is managed with long term anticoagulation therapy that is associated with an increased bleeding risk. Current understanding of APS indicates that the aPLs produced by B lymphocytes promote clot formation in several ways: 1) induce procoagulant phenotype in endothelial cells, 2) inhibit anticoagulant proteins, 3) upregulate leukocyte adhesion, 4) enhance neutrophil extracellular trap (NET) release. Our recent studies established activated neutrophils as key determinants of both arterial and venous thrombosis in APS. Further, we demonstrated that transcription factor Krüppel-like factor 2 (KLF2) is a key modulator in neutrophil activation. Genetic loss of KLF2 and neutrophil activation leads to P-selectin glycoprotein ligand 1 (PSGL-1) clustering on neutrophil uropods facilitating neutrophil-endothelial cell adhesion and exacerbation of both venous and arterial thrombosis. We have also shown that a nanomedicine targeting such PSGL-1 clustering using designer immuno-nanoparticles alleviates thrombosis in KLF2-/- mice and mice exposed to aPLs.
Here, we hypothesized that a fine tuning of a nanoparticle shape and surface chemistry may improve selective targeting of activated neutrophils and further refine nanomedicine approach towards thrombosis in APS. Using primary neutrophils in culture, we found that the shape of anti-PSGL-1 antibody-coated gold nanoparticles (aPSGL-AuNPs) greatly influenced the efficacy of nanoparticle-neutrophil interaction. Screening of a small library of diverse aPSGL-AuNPs for blockade of activated neutrophil attachment has demonstrated that star-shaped aPSGL-AuNPs★ were at least 4-6 fold more efficacious, compared to other shapes of nanoparticles. Under transmission electron microscopy (TEM), we observed that the aPSGL-AuNPs★ revealed greater affinity to uropods on activated neutrophils, co-localizing with highly-segregated PSGL-1. In APS-induced laser-injury carotid artery and complete inferior vena cava (IVC) ligation deep vein thrombosis (DVT) models aPSGL-AuNPs★ prolonged occlusive thrombosis time (35土2.2 vs. 18土2.4 min, aPSGL-AuNPs★ vs. IgG-AuNPs★, P=0.00042) and lessened thrombus weight (27.5土3.2 vs. 37.75土1.8 mg, aPSGL-AuNPs★ vs. IgG-AuNPs★, P=0.033) respectively. Further, in aPSGL-AuNPs★-treated mice, thrombus from the DVT model were almost devoid of neutrophils and NETs as compared to control IgG-AuNPs★-injected animals. Finally, aPSGL-AuNPs★ treatment did not alter tail bleeding times or complete blood counts as compared to IgG-AuNPs★.
Collectively, these findings reveal an exciting novel for targeting activated neutrophils in vascular thrombosis. Our studies demonstrate that targeting is dependent on the specific topographical features of the highly-segregated PSGL-1 on the activated neutrophil surface, for which a high affinity shape-driven nanomedicine can be designed and implemented. As such, aPSGL-AuNPs★ serve as a promising nanoimmunotherapy for immunothrombosis associated with neutrophil adhesion and NET formation noted in APS.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal